Marie Sklodowska Curie - Appendices
Appendices
1) The partitions of Poland 1772-1795
The kingdom of Poland had many weaknesses. The nobles elected its kings. The election campaigns to determine who would be king invited domestic and international troubles. Prussia, Austria, France and Russia each repeatedly plotted to put its favorite on the Polish throne. Only nobles were represented in Poland's legislature, the Diet. The Diet rarely accomplished anything because any one member could veto any legislation being considered.
Poland contained large minority groups of various nationalities and religions. The Roman Catholic Poles and their leaders often discriminated against and oppressed the minorities, and sometimes they appealed to Prussia, Austria or Russia for help.
In 1772 these three powers decided to take advantage of Poland's weak condition and seize a slice of Polish territory. This action is known as the First Partition of Poland....
In 1793 Russia and Prussia took a second helping of Polish lands. The rebellion that broke out over this Second Partition was crushed but it brought about the Third Partition in 1795 by Austria, Prussia and Russia. With that, Poland disappeared from the map of Europe until 1919.1
______________________
1 Extract From 'World History' People and Nations by Anatole G. Mazour, John M. Peoples, HBJ Harcourt Brace Jovanovitch Publishers, Orlando, Florida 1990, page 387.
2) Science laboratory
"If the conquests useful for humanity touch your heart, if you are overwhelmed before the astonishing results of electric telegraphy, of the daguerreotype, of anesthesia, and of other wonderful discoveries, if you are jealous of the part your country may claim in the spreading of these marvelous things, take an interest, I beg of you, in those sacred places to which we give the expressive name of laboratories. Demand that they be multiplied and ornamented, for these are the temples of the future, of wealth, and of well-being. It is in them that humanity grows, fortifies itself, and becomes better. There it may learn to read in the works of nature the story of progress and of universal harmony, even while its own creations are too often those of barbarism, fanaticism, and destruction."1
"It is useful to learn how much sacrifice such a life represents. The life of a great scientist in his laboratory is not, as many may think, a peaceful idyll. More often it is a bitter battle with things, with one's surroundings, and above all with oneself. A great discovery does not leap completely achieved from the brain of the scientist, as Minerva sprang, all panoplied, from the head of Jupiter; it is the fruit of accumulated preliminary work. Between the days of fecund productivity are inserted days of uncertainty when nothing seems to succeed, and when even matter itself seems hostile; and it is then that one must hold out against discouragement. Thus without ever forsaking his inexhaustible patience, Pierre Curie used sometimes to say to me: 'It is nevertheless hard, this life that we have chosen.' "2
_____________________________
1 Louis Pasteur.
2 Marie Curie,Autobiographical Notes.
3) Radioactivity
Radioactivity is the emission of energetic particles or waves from atoms. In 1896, Henri Becquerel observed that the first radiation of unknown origin was emitted by uranium salts. Several materials other than uranium were also found to emit these penetrating rays. Materials that emit this kind of radiation are said to be radioactive and to undergo radioactive decay.
The phenomenon of radioactivity is very difficult to observe as it is produced deep within the atom, in its very nucleus. Matter is composed of atoms, most often assembled in molecules. An atom consists of an extremely small, positively charged nucleus surrounded by a cloud of negatively charged electrons. Although typically the nucleus is less than one ten-thousandth the size of the atom, the nucleus contains more than 99.9% of the mass of the atom! Nuclei consist of positively charged protons and electrically neutral neutrons held together by the so-called strong or nuclear force. This force is much stronger than the familiar electrostatic force that binds the electrons to the nucleus, but its range is limited to distances on the order of a few x 10-15 meters.
Certain unstable atom nuclei are the source of radiation, designated by the first three letters of the Greek alphabet: alpha (α), beta (β) and gamma (γ). This radiation is composed of particles emitted by high energy nuclei. Alpha radiation is composed of light helium nuclei, β radiation of positive or negative electrons and γ radiation of high energy photons.
If nuclei come close enough together, they can interact with one another through the strong nuclear force, and reactions between the nuclei can occur. As in chemical reactions, nuclear reactions can either be exothermic (i.e. release energy) or endothermic (i.e. require energy input). Two major classes of nuclear reactions are of importance: fusion and fission.
Radioactivity is a natural phenomenon which surrounds us. All

Marie Curie in her laboratory

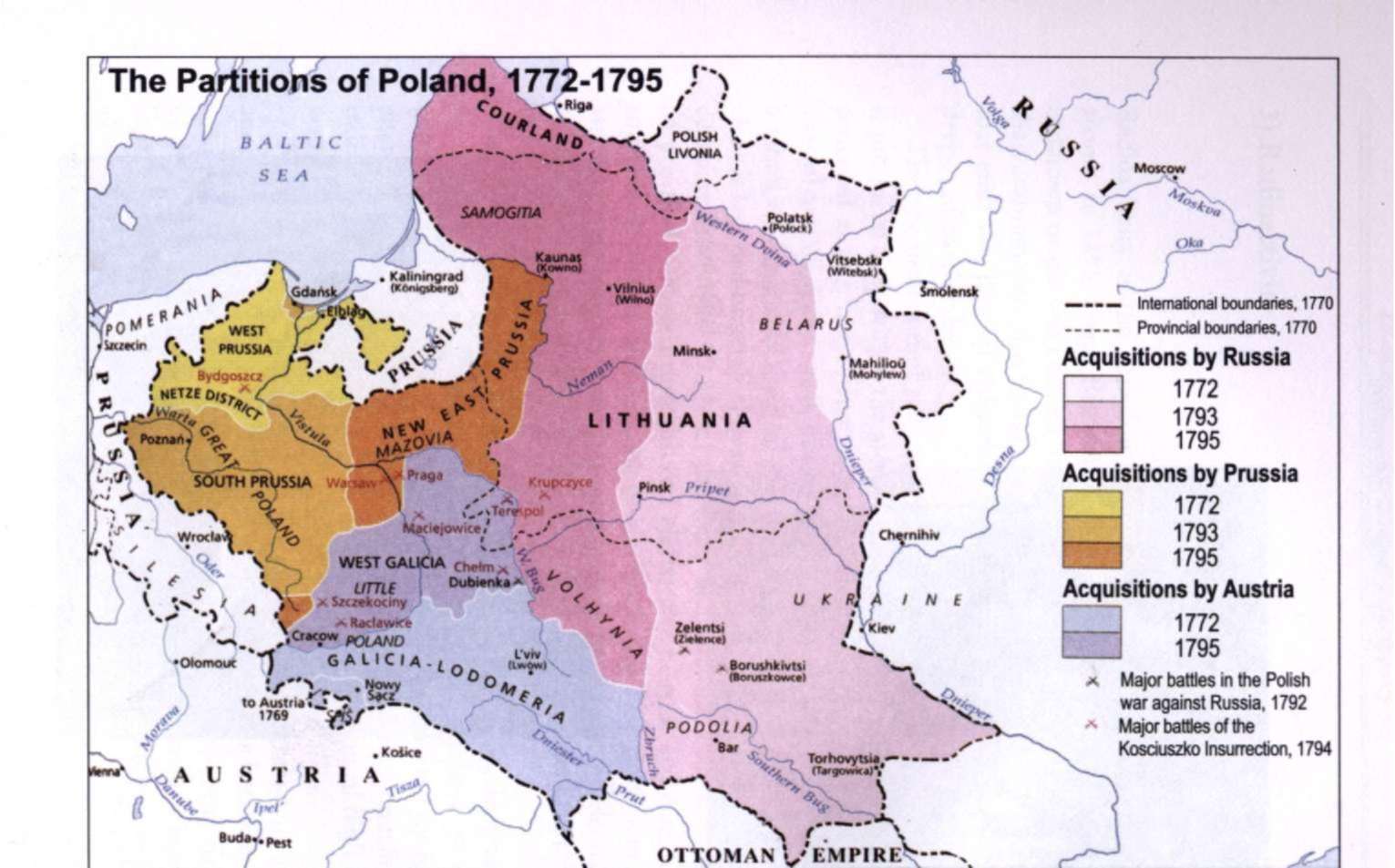
When Marie was born in 1867, Poland did not exist as an independent nation. Between 1772 and 1795 the entire territory of the Kingdom of Poland was divided between three powerful empires: Prussia, Austria and Russia.
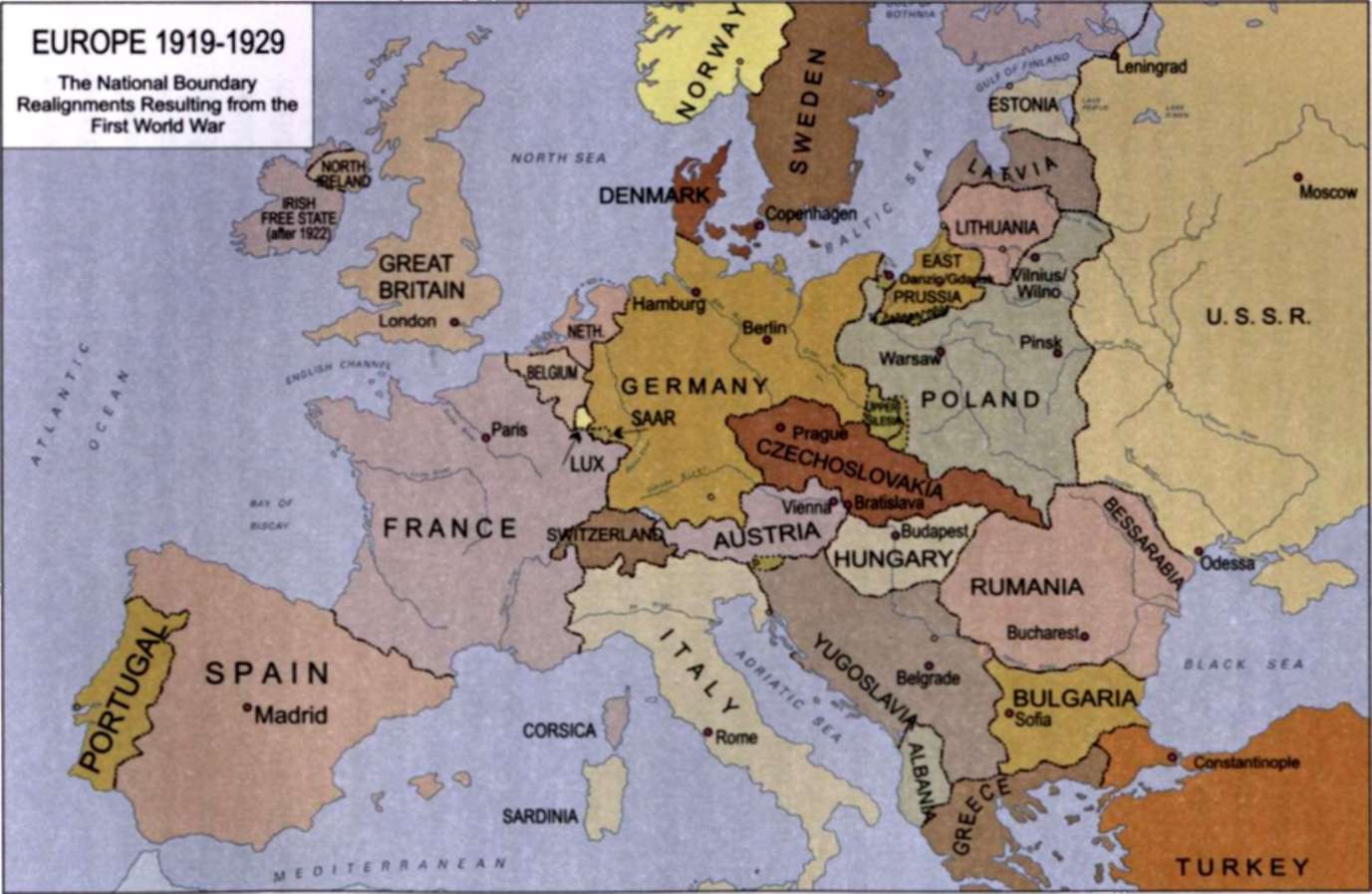
After the first World War, "1 had lived, though I had scarcely expected it, to see the reparation of more than a century of injustice that had been done to Poland, my native country..."
the energy we get from the sun comes from one comparatively simple reaction: the fusion of two hydrogen nuclei into another, heavier nucleus. We know that all life on Earth exists because the light generated by the Sun produces food and warms our planet. Therefore, we can say that fusion is the basis for our life This reaction is what has allowed elements other than hydrogen to come into existence. Without it, the great matter factories of the stars would not be able to build the heavier elements that make up our universe.
Without radioactivity, our planet would have frozen over long ago and life on earth would not have been possible. Radioactive processes in the earth's core slowly release the heat essential for our survival, constantly maintaining the temperate climate we take for granted. All life has developed in a constant shower of radiation, adapting to it and occasionally using it for its own benefit.
Our planet is a warm one, but the pleasant conditions we enjoy on its surface are principally due to the radioactive processes taking place at its centre. The earth's radioactivity causes our planet to behave like an immense hot-water bottle, slowing down the cooling rate and consequently making it habitable. The heat necessary for our survival is released by the radioactive disintegrations which take place in the rocks that form our earth's crust.
As with fusion, a great amount of energy can be released in fission. Fission is a nuclear process in which a heavy nucleus splits into two smaller nuclei. The fission of the atom nucleus is a natural phenomenon that releases, at the atom scale, millions times more energy than in the other sources of energies used by mankind. An example of a fission reaction is one that was used in the first atomic bomb and is still used in reactors. Fission is a process that has been occurring in the universe for billions of years. As mentioned above, we have not only used fission to produce energy for nuclear bombs, but we also use fission peacefully everyday to produce energy in nuclear power plants. Interestingly, although the first man-made nuclear reactor was produced only about fifty years ago, the Earth operated a natural fission reactor in a uranium deposit in West Africa about two billion years ago!
Radiation is an omnipresent, inescapable feature of our lives.
Wherever we go we will always be exposed to it. Even our bodies are radioactive, containing as they do millions of atoms of radioactive substances such as potassium.
The large diversity of radioactive elements together with the tremendous spectrum their respective half-lives lead to many applications. Some can survive for billions of years, while others disappear within minutes. This range, from the quasi-permanent to the instantaneous, allows for radioactivity to be used in practically every sphere of human endeavor.
Radioactive sources were what allowed Ernest Rutherford, Marie Curie and others to conduct the first examinations of the nucleus. In the intervening century, radioactivity has revealed itself capable of a myriad of applications, and has become an invaluable source of subatomic information. The very nature of radioactivity allows scientists to trace atoms or molecules with great sensitivity. The discovery of artificial radioactivity in 1934 gave scientists the ability to create radioactive isotopes of any element they wanted. Apart from the countless natural sources of exposure, the harnessing of radiation by humanity has led to a multitude of applications that we use every day. The main artificially generated exposure comes from medical procedures (such as X-rays) but we use radioactive substances and radiations to sterilize food, prolong its shelf life, and prevent fires in public places. However, all these common sources of radiation, whether natural or artificial, remain virtually harmless.
Applications of Radioactivity
One of the more important applications of this technology was the development of radioactive tracers, which have helped to revolutionize biology. Using radioactive tracers, it is now possible to understand the way atoms and molecules move through our bodies. In biological research, short-lived radioactive tracers such as tritium or phosphorus 32 are universally popular. An element such as iodine 123 allows for the examination of thyroid problems with a gamma camera. Those methods have led to a better understanding of the human metabolism and provided an excellent way to verify the effect of drugs
and medication.
The harnessing of radioactivity has also led to the development of one of the most important tools in the medical arsenal. Positron Emission Tomography (also known as PET scanning) gives doctors the ability to film the inner workings of the brain, and to detect cancers in the early stages of development. Such techniques, however, are still expensive, and consequently fairly uncommon. More widespread is the process of 'scintigraphy' - the imaging of gamma radiation with so-called 'gamma cameras'. Scintigraphy scans can be conducted on bones, the thyroid gland, kidneys, lungs, or even on the muscles of the heart. The risks patients face from such radioactive scans are minimal when compared to the benefits such tests can offer.
Another application of Radioactivity relates to our cultural heritage and dating. Carbon 14, with its half-life of 5.700 years, has revolutionized the field of archaeological dating. Using the mathematical laws underlying all radioactive decay, objects and places up to 40.000.years old can be accurately dated. Other radioactive techniques (such as examining the ratio between quantities of potassium and argon present in a sample) can be used to date much older rocks.
Nuclear techniques are used by museographers. Before being exhibited in a museum, the vestiges of past civilisations must be identified and analyzed. The authenticity of the objects is guaranteed by these analyses, which can also supply as much information as possible on their archeological and historical origins.
Here are several examples of application: The ashes which were found in the Chauvet grotto have been analyzed. Their content of carbon-14 has helped to date the period in which the grotto was inhabited by prehistoric men as long as 35000 years ago. A medieval wooden statue was coated with a resin which was hardened by radiation. Thanks to this treatment it can finally be shown to and admired by the public. At the Carnavalet Museum in Paris, Neolithic dugouts found along the banks of the Seine are exposed. One of them was consolidated by radiation, so there is no more risk of it falling to dust. The mummy of a great pharaoh travelled from Cairo to Paris. Before returning to its country it was radiated by gamma rays in
order to destroy all the micro-organisms which were devouring it. The paintings exhibited in the Louvre are not copies. Aglae, a small accelerator in the Center for research and restoration in French museums, has guaranteed their authenticity.
Radioactivity also has uses in earth science and the study of the environment. It was in these fields that uranium, potassium and rubidium allowed for an accurate estimate of the age of the earth. Climatologists measure solar activity by seeing how much beryllium 10 produced by cosmic rays gets absorbed by the polar ice caps. Oceanographers can, through measuring the abundance of carbon 14 in the oceans, retrace the flows of ancient currents.
Our fear of radiation dates back to the early 20th Century, when many of the early experimenters with radioactivity started to feel the negative consequences of the exposure on their bodies. Much later, in 1945, the use of the atomic bombs on Hiroshima and Nagasaki clarified the enormous risks that a misuse of radioactivity could entail. The 1986 Chernobyl disaster also did much to prejudice us against this powerful natural phenomenon.
Our fear is inextricably tied up with the mysterious and insidious nature of these invisible rays. What is the exact relationship between exposure and future risk? Is there a threshold below which radiation is truly harmless? In all such cases and for all such questions, there is no clear answer. The effects of exposure to radiation depend heavily on the nature of the exposure, which organs were exposed and the general health of the person at the time of the incident.
In order to set up levels permissible for living beings, it is of importance to define doses of exposure which take into account the sensitivity of the whole human body, or in medicine, of a particular organ. This is precisely where radioprotection comes in. Radioprotection is the study of such risks, and the basis of all our attempts to minimize them. Over the last hundred years, the field of radioprotection has devised regulations and safety procedures specifically. The fundamental principle of precaution is that the potential risk may be never zero and that it should be minimized as much as possible.
Nuclear power also generates wastes that are small in volume but highly radioactive and whose management necessitates the greatest
care. Among all the radioactive materials that the ordinary citizen has to be shielded from, the ones that preoccupy him the most are the ones originating from nuclear plants, since they are by far the more radioactive, eclipsing by their activity all the materials coming from hospitals, laboratories and industry. These matters are not ordinary wastes as they contain plutoniurn, a highly strategic element. The retrieval or not of plutonium is at the core of the management of the spent fuel of reactors. The progresses of waste management are impressive since the not so far away prehistory of nuclear power when radioactive packages were dropped into the sea. The level of the confinement of radioactivity achieved today is quite remarkable and probably already sufficient. Past and ongoing researches aim to reduce further the volume and harmfulness of radioactive matters.

Marie Curie in her laboratory with husband Pierre
4) Milestones on the path of scientific progress
From 1996 to 1998, the centennial of the discovery of radioactivity was celebrated in France. Expositions were held, meetings took place, films were shown and symposia were organized. The opportunity was also taken to inform the general public of the consequences of this major discovery, the improved understanding of the atomic structure that resulted from it and the improvements to our quality of life that understanding has brought.
At the turn of the last century and before the discoveries of radioactivity, the atom was an entirely hypothetical concept, whose very existence was doubted in the scientific community.
It was only in 1906 that the French physicist Jean Perrin was conclusively able to demonstrate the existence of the atom. Marie Curie and her husband Pierre had been curious about what was happening inside the atom, and postulated that radioactivity was an atomic phenomenon. In a world where the atom was held to be the fundamental, indivisible unit of matter, the proposal that an atom was capable of decaying or of transforming amounted to heresy.
The following decades saw very many great experimentalists attempting to discover the true nature of the atom. Using the newly-discovered phenomenon of radioactivity, they were able to deduce that an atom consisted of an extremely dense central nucleus surrounded by orbiting electrons. Further experimentation revealed even more fundamental units of matter: the nucleus consists of neutrons and protons bound together very tightly, and both neutrons and protons are comprised of even smaller "quarks". At around the same time, physicists proposed the existence of a particle known as the 'neutrino'for which no direct evidence existed, but whose presence made the physics work. The neutrino would be discovered, exactly as predicted.
All this work being done on the atom revealed the existence of a fundamental force that had previously gone unnoticed before the discovery of beta radioactivity. The weak interaction is seen today as a force that occurs between particles when they exchange 'W' or
'Z' bosons. Experiments at CERN provided in 1982 evidence for these hypothesized particles. The question asked some 80 years ago was finally answered: "Where does radioactivity come from?" In parallel to all of these experimental discoveries, a large number of possible applications of radioactivity were revealed. These ranged across all fields, from the generation of energy to the treatment of cancer to the production of weaponry. The harnessing of the power of radiation is undoubtedly one of humanity's greatest intellectual achievements.

This photograph is from the famous 5th Solvay Conference in Belgium (October 1927), which brought together the greatest minds of the last century including Einstein, Curie, Schroedinger, Bohr, Heisenberg, Planck, Dirac, Pauli, Lorentz, Born, etc.
The majority of the twenty-nine attendees are Nobel Prize winners.
The conference was dedicated to quantum theory.
5) Marie's Firsts
Perhaps the most famous of all women scientists, Maria Sklodowska-Curie is notable for her many firsts:
- She was the first to use the term radioactivity for this phenomenon.
- She was the first woman in Europe to receive her doctorate of science.
- In 1903, she became the first woman to win a Nobel Prize for Physics. The award, jointly awarded to Curie, her husband Pierre, and Henri Becquerel, was for the discovery of radioactivity.
- She was also the first female lecturer, professor and head of Laboratory at the Sorbonne University in Paris (1906).
- In 1911, she won an unprecedented second Nobel Prize (this time in chemistry) for her discovery and isolation of pure radium and radium components. She was the first person ever to receive two Nobel Prizes.
- She was the first mother-Nobel Prize Laureate of a daughter-Nobel Prize Laureate. Her oldest daughter Irene Joliot-Curie also won a Nobel Prize for Chemistry (1935).
- She is the first woman which has been laid to rest under the famous dome of the Pantheon in Paris for her own merits.
- She received 15 gold medals, 19 degrees, and numerous other honors.

La Sorbonne University, Paris

Pierre Curie
6) A Tribute to Pierre Curie by Marie Curie

Pierre, Marie and Irene
"I have attempted to evoke the image of a man who, inflexibly devoted to the service of his ideal, honored humanity by an existence lived in silence, in the simple grandeur of his genius and his character. He had the faith of those who open new ways. He knew that he had a high mission to fulfill and the mystic dream of his youth pushed him invincibly beyond the usual path of life into a way which he called anti-natural because it signified the renunciation of the pleasures of life. Nevertheless, he resolutely subordinated his thoughts and desires to this dream, adapting himself to it and identifying himself with it more and more completely. Believing only in the pacific might of science and of reason, he lived for the search of truth. Without prejudice or parti pris, he carried the same loyalty into his study of things that he used in his understanding of other men and of himself. Detached from every common passion, seeking neither supremacy nor honors, he had no enemies, even though the effort he had achieved in the control of himself had made of him one of those elect whom we find in advance of their time in all the epochs of civilization. Like them he was able to exercise a profound influence merely by the radiation of his inner strength."
"Marie had never had time to be a perfect educator to her daughters. But Irene and Eve received one gift from her that they will never be able to appreciate enough: the incomparable benefit of living near an exceptional being, exceptional not only in her genius but by her humanity, by her innate refusal of all vulgarity and littleness. Mme Curie avoided even that element of vanity that might most easily have been forgiven her: to let herself be cited as an example to other women."1

Eve Curie speaking with Theodore Roosevelt Jr. and Dr. Stecker during the party for the publication of her book Madame Curie.
_____________________
1 Eve Curie

Frédéric and Iréne joliot Curie
Iréne Curie (1897-1956)
Irene, born in Paris, 12 September, 1897, was the first daughter of Pierre and Marie Curie. She began her studies at the Faculty of Science in Paris and during the First World War she served as a nurse radiographer. She became a Doctor of Science in 1925, and married Frédéric Joliot in 1926. Iréne and Frédéric shared years of effort and scientific passion as before them Pierre and Marie Curie.- She worked on natural and artificial radioactivity,
transmutation of elements, and nuclear physics in collaboration with her husband with whom she shared the Nobel Prize in Chemistry for 1935 in recognition of their synthesis of new radioactive elements.
In 1936 Iréne Joliot-Curie was appointed Undersecretary of State for Scientific Research. She became Professor in the Faculty of Science in Paris in 1937. In 1938 her research on the action of neutrons on the heavy elements, was an important step in the discovery of uranium fission.
In 1946 she became Director of the Radium Institute. Being a Commissioner for Atomic Energy for six years, Irene took part in its creation and in the construction of the first French atomic pile (1948). She was involved in the building of the large centre for nuclear physics at Orsay for which she worked out the plans.
She took a keen interest in the social and intellectual advancement of women; she was a member of the National Committee of the Union of French Women and of the World Peace Council. She was a Frederic and Irene Joliot Curie
member of several foreign academies and of numerous scientific societies, had honorary doctor's degrees from several universities, and was an Officer of the Legion of Honour.
Irene died in Paris in 1956. Frederic and Irene Joliot-Curie had one daughter, Helene, born in 1927, and one son, Pierre, born in 1932. Pierre is a biochemist. Helene is a nuclear physicist and professor at the University in Paris; she married Paul Langevin's grand-son: Michel Langevin (1926-1985) who was a physician; their son Yves Langevin is an astrophysicist. (Paul Langevin was a French physicist who worked with Pierre Curie.)1
8) Eve Curie (1904-2007)
Eve Curie was born in Paris on 6th December 1904 as Pierre and Marie Curie's youngest child. She was a gifted musician as was her grandmother Bronislawa, Marie Curie's mother. Marie always encouraged her to develop her skill and she first had a career as a concert pianist. Later on she became a writer and a journalist during World War II.
The biography she wrote after her mother's death was a best seller in 80 countries. She married Henri Labouisse (1904-1987), a diplomat who became President of UNICEF and was awarded the Nobel Peace Prize in 1965. Eve Curie lived in New York where she died on October 26th 2007 at the age of 103. She used to say while joking: "I am the shame of my family. There were five Nobel Prize among mine, my mother got two, my father one, my sister and her husband one, even my husband had one, and it has only me there who did not receive any!"
* * *
____________________________
1. Excerpted from Nobel Lectures, Chemistry 1922-1941, Elsevier Publishing Company, Amsterdam, 1966 .
9) Chronology
| 1859, May | 15 Birth of Pierre Curie in Paris. |
| 1867, Nov. 7 | Birth of Marya Sklodowska in Warsaw. |
| 1891, Nov | Marie arrived in Paris. |
| 1895, July | Pierre Curie married Marie Sklodowska. |
| 1895,Nov. | Discovery of X-rays by Wilhelm C. Roentgen in Germany. With radiography it was possible for the first time to look inside the human body |
| 1896 | At the Museum of Natural History, Henri Becquerel discovered that the element uranium emits penetrating radiation (natural radioactivity). |
| 1896,July | First treatment of cancer using X-rays. |
| 1897 | J.J. Thomson characterized the electron by measuring its speed and charge-to-mass ratio, and showed that all atoms contain electrons. |
| 1897,Sept.12 | Birth of Iréne Curie, first daughter of Pierre and Marie. |
| 1898 | Pierre and Marie Curie discovered polonium (July) and radium (December) at the School of Industrial Physics and Chemistry (EPCI) in Paris. Marie Curie coined the term radioactivity. |
| 1899 | Several scientists demonstrated that uranium emits two types of radiation. In January, Ernest Rutherford called them respectively a and ß radiation. On 6 November, Pierre and Marie Curie published a paper in which they reported a singular property of radium: "induced radioactivity". |
| 1900, March 19 | Birth of Frédéric Joliot |
| 1901-1904 |
Jean Perrin (and independently Hantaro Nagaoka) assumed that the atom had a structure reminding to that of the solar system. |
| 1901-1903 | Ernest Rutherford and Frederic Soddy demonstrated the "period" (according to the law of radioactive decay) that characterizes each radioactive element. They showed that radioactivity is the transmutation of one element into another. |
| 1903, Dec. |
Pierre and Marie Curie were awarded the Nobel Prize in Physics with the French scientist Henri Becquerel. |
| 1904 | Pierre Curie became a professor at the University of the Sorbonne. |
| 1905 | Recognition of the beneficial action of radium rays in treating tumors. Birth of Curie therapy. |
| 1906, April 19 | Pierre Curie died in an accident. |
| 1906, Nov. | Marie Curie became the first woman to teach at the Sorbonne Ernest Rutherford identified a radiation as a helium particle. |
| 1909 | Decision taken to build the Radium Institute. |
| 1910 | Marie Curie isolated metallic radium and determined its atomic mass. |
| 1911 |
Frederic Soddy established the existence of isotopes. Ernest Rutherford demonstrated the presence of a nucleus at the center of the atom. Marie Curie was awarded the Nobel Prize in Chemistry. |
| 1913 |
Niels Bohr worked out a model of the atom in which electrons orbit the nucleus. |
| 1914-1918 |
First World War. Marie Curie equipped vehicles with radiology equipment, called 'Little Curies", for treatment of the wounded at the warfront. |
| 1919 | Ernest Rutherford performed the first artificial nuclear transmutation, by transforming nitrogen into oxygen through particle bombardment. Opening of the Radium Institute in Paris. |
| 1920 | Creation of the Curie Foundation. |
| 1924 | Frederic Joliot works as Marie Curie's assistant at the Radium Institute. He will marry Irene Curie in 1926. |
|
1930 |
Ernest Lawrence built the first cyclotron at Berkeley. |
|
1932 |
James Chadwick demonstrated the existence of the neutron. |
|
1934 |
Frédéric and Irene Joliot-Curie discovered artificial radioactivity. A radioactive element was created for the first time. The discovery of artificial radioactivity heralded the emergence of nuclear medicine |
|
1934, July 4 |
Marie Curie died from leukemia. |
|
1935 |
Irene and Frederic Joliot-Curie awarded the Nobel Prize in Chemistry for the discovery of radioactivity. |
|
1936 |
Opening of the Curie Foundation hospital in Paris. |
|
1938: |
Otto Hahn and Fritz Strassmann reported the fission of uranium |
|
1939 |
Frederic Joliot, Hans Halban, Lew Kowarski and Francis Perrin demonstrated the possibility of the chain reaction and therefore of its energy applications. They showed that fission is accompanied by great release of energy and by the emission of neutrons which can break open other uranium nuclei, and so on. |
|
1956 |
Irene Curie died from leukemia. |
|
1958 |
Frederic Joliot-Curie died in Paris. |
10) Curie dictionary-
Curie: (symbol Ci) is the old standard unit for measuring the activity of a given radioactive sample. It is equivalent to the activity of 1 gram of radium.
Curietherapie: medical application of the use of radium in radiation therapy.
Little Curies or Petites Curies: During the First World War in 1914, to avoid transporting the wounded away from the front, Marie Curie outfitted cars. About 200 vehicles were equipped with X-ray equipment and sometimes with a generator. At the battle-front, in makeshift hospitals with the help of X-ray examinations, doctors were able to locate bullets and shells. In this way approximatively one million examinations were conducted. It was the first medical imagery service in France.
Curium: a synthetic radioactive chemical element with the atomic number 96, discovered in 1944, named after Pierre and Marie Curie.
Curite: radioactive mineral named after Pierre Curie.
Sklodowskite and Cuprosklodowskite: radioactive elements named after Marie Sklodowska-Curie.
Curie Institute or Institut Curie: a world center in Paris for the study of radioactivity, one of the most prestigious research institutions for research on and treatment of cancer.
Marie Curie Fellowship Association: association of scientists (Marie Curie Fellows) who were awarded mobility research training grants by the European Community.
Curie Museum: Located at the Curie Institute in Paris, this museum was housed in the same laboratory where Marie worked up to her death.
Pierre and Marie Curie University: name of one of the faculties of Sciences of Paris.
Maria Curie-Sklodowska University: name of a public university in Lublin, Poland.
Station Pierre et Marie Curie: name of a Paris metro station.

French postal stamp (1938)
The inscription reads: Pierre and Marie Curie discover radium, Nov. 1898.
International Union Against Cancer
11) General notes
Atom: The smallest component of matter which cannot be broken down by any chemical means. A typical atom consists of a nucleus of protons and neutrons with electrons circling this nucleus.
Claude Bernard (1813-1878): French physiologist who defined the fundamental principles of scientific research in his treatise: An introduction to the Study of Experimental Medicine.
Auguste Comte (1798-1857): French philosopher, who was considered as one of the founders of sociology (the study of human social behavior). His school of thought, Positivism, was influential towards the end of the 19th century. Positivism holds that the only authentic knowledge is that based on actual sense experience.
Cyclotron: A type of particle accelerator. A cyclotron accelerates charged subatomic particles (protons, electrons) greatly increasing their energy.
Charles Darwin (1809-1882): British naturalist and biologist, author of a theory of organic evolution claiming that new species arise and are perpetuated by natural selection, known by the name of Darwinism.
Don Quixote: Tragicomic hero of a Cervantes novel. Don Quixote's main quest in life is to revive chivalric virtues and values. Honest and idealistic, he wants to save the world and dispense justice. He is the symbol of an absurd dreamer living in his own world.
Isotope: One or two or more atoms having the same atomic number but different mass numbers. Isotopes are different forms of a
single element. For example, Carbon 12 and Carbon 14 are both isotopes of carbon, one with 6 neutrons and one with 8 neutrons but both with 6 protons.
Alfred Nobel (1833-1896): Chemist, engineer, inventor of dynamite and manufacturer of explosives. Born in Sweden. When his brother died, a French journalist published his obituary by mistake and called him a 'merchant of death'. Realizing the disaster that his inventions were causing, Alfred Nobel wrote his last will and testament and established the Nobel Foundation to administrate the Nobel Prizes. Every year since 1901, in physics, chemistry, medicine, literature and work for peace, men and women, without distinction of nationality are recognized for their outstanding contributions in their field of work.
Louis Pasteur (1822-1895): French chemist and biologist. His most celebrated work was related to the discovery of a method, pasteurization, for the preservation of food. By this method, harmful microorganisms are destroyed with high temperature without any major changes in the chemistry of the food. He produced the first vaccine against rabies.Pantheon: Monument in Paris. Originally a church, it became a burial place for famous French heroes. The inscription above the entrance reads: "Aux grands hommes, la patrie reconnaissante" ("To its great men, the grateful homeland.") The absence of a verb in French emphasizes that the implicit notion of honour is given from the homeland to the great men. By burying its great men in the Pantheon, the nation acknowledges the honor it received from them.
Oswald Spencer (1820-1903): British philosopher and sociologist, who was one of the principal proponents of evolutionary theory in the mid-nineteenth century. At the time, his reputation rivaled that of Charles Darwin.
Radioactivity: name given by Marie Curie, in 1898, to the atomic property of certain heavy elements which spontaneously emit radiation; this property is persistent in all physical and chemical states of matter.
The discovery of radioactivity brought several means to study the constitution of the atom and atomic nucleus. Today, radioactivity and X-rays are employed in medicine in general, in archaeology, geology, in the restoration of art works and for food conservation.
Tsar or Czar: Russian imperial title in use until the revolution in 1917, derived from the Latin Caesar, the title of Roman Emperor.
X-Rays: One century ago, Wilhelm Konrad Roentgen discovered the X-ray (so called because at that time no one knew what this was) which began the use of energy to visualize medical problems in patients. The subspecialty of medicine which developed from this discovery is Radiology. With X-rays both then and now, the rays themselves (a form of energy) are not visible with the eye. Another method or material must be used to convert the information to a visible or useable form. X-rays typically use film or screens combined with TV to make the structures penetrated by ray visible.
* * *
12) Suggestions for further reading
- Curie, Eve. Madame Curie. Translated by Vincent Sheean. 1937. Reprint, New York: Da Capo, 1986.
- Curie, Marie. Pierre Curie. Translated by Charlotte and Vernon Kellogg. New York: Macmillan, 1932. (Marie Curie's Autobiographical Notes are included at the end of Pierre Curie.)
- Curie, Marie. CEuvres de Marie Sklodowska-Curie. ac. Pol. Sci. Varsovie: 1954. New York and London: W. W. Norton & Company, 2005.
- Curie, Pierre. CEuvres de Pierre Curie. Gauthier Villars, 1908
- Giroud, Frangoise. Marie Curie: A Life. Translated by Lydia Davis. New York: Holmes & Meier, 1986.
- Goldsmith, Barbara. Obsessive Genius: The Inner World of Marie Curie.
- 'McGrayne, Sharon Bertsch. Nobel Prize Women in Science: Their Lives, Struggles, and Momentous Discoveries. New York: Carol Press, 1998.
- Pasachoff, Naomi. Marie Curie and the Science of Radioactivity. New York and Oxford: Oxford University Press, 1996.
- Pflaum, Rosalynd. Grand Obsession: Madame Curie and Her World. New York: Doubleday, 1989.
- Quinn, Susan. Marie Curie: A Life. New York: Simon & Schuster, 1995.
- Reid, Robert William. Marie Curie. New York: New American Library, 1978.
- Woznicki, Robert. Madame Curie - Daughter of Poland. Miami: American Institute of Polish Culture, 1983.
Related Books
- Alexander the Great
- Arguments for The Existence of God
- But it is done
- Catherine The Great
- Danton
- Episodes from Raghuvamsham of Kalidasa
- Gods and The World
- Homer and The Iliad - Sri Aurobindo and Ilion
- Indian Institute of Teacher Education
- Joan of Arc
- Lenin
- Leonardo Da Vinci
- Lincoln Idealist and Pragmatist
- Marie Sklodowska Curie
- Mystery and Excellence on The Human Body
- Nachiketas
- Nala and Damayanti
- Napoleon
- Parvati's Tapasya
- Science and Spirituality
- Socrates
- Sri Krishna in Brindavan
- Sri Rama
- Svapnavasavadattam
- Taittiriya Upanishad
- The Aim of Life
- The Crucifixion
- The Good Teacher and The Good Pupil
- The Power of Love
- The Siege of Troy
- Uniting Men - Jean Monnet